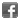Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
3,3'-Diethylthiadicarbocyanine iodide
| Product Details | |
|---|---|
| Synonyms | DiSC2(5); 3-Ethyl-2-[5-(3-ethyl-2(3H)-benzothiazolylidene)-1,3-pentadienyl]benzothiazolium iodide; DTDCI; Dithiazine; SI 1964 |
| Product Type | Chemical |
| Properties | |
| Formula | C23H23IN2S2 |
| MW | 518.48 |
| CAS | 514-73-8 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Dark green to black powder. |
| Solubility | Soluble in DMSO. Slightly soluble in methanol or ethanol. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | MNQDKWZEUULFPX-UHFFFAOYSA-M |
| Smiles | CCN1C2=CC=CC=C2S/C1=C/C=C/C=C/C3=[N+](CC)C4=CC=CC=C4S3.[I-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
3,3'-Diethylthiadicarbocyanine iodide (DiSC2(5); DTDCI), is a fluorescent near-infrared (NIR) dye. It belongs to the family of carbocyanine laser dyes. Due to its NIR fluorescence, it is used in various imaging techniques to visualize cells and tissues, and it is often used to study membrane potentials in cells because its fluorescence properties can change in response to membrane potential variations. DTDCI has strong absorption in the near-infrared region λex = ~660nm. This region is particularly useful for biological applications as it allows for deeper tissue penetration and minimizes autofluorescence from biological samples. DTDCI emits fluorescence in the near-infrared (NIR) region. The exact λem max typically occurs ~750-800nm, though this can vary slightly depending on the solvent and environment. The Stokes shift for DTDCI is relatively large, which is advantageous as it helps in reducing background interference and improving the signal-to-noise ratio in imaging applications. DTDCI, like many carbocyanine dyes, can be prone to photobleaching, where prolonged exposure to light causes a reduction in fluorescence intensity. Spectral data: λex max = 651 nm. DTDCI is a monocationic dye which forms cofacial dimers that insert into the minor groove of DNA. It is also an effective broad-spectrum anthelmintic. DTDCI is used as a fluorescent contrast agent in three-dimensional fluorescence lifetime tomography and as a dye for rapid genetic screening. DTDCI is used as a tracer in cell biology. The dye's mechanism of action involves its ability to intercalate into lipid bilayers and selectively stain mitochondria, which may be useful for studying mitochondrial dynamics and function.
(1) F.P. Schaefer, et al.; Appl. Phys. Lett. 9, 306 (1966) | (2) D.N. Dempster, et al.; J. Chem. Soc. Faraday Trans. 2, 68 (1972) | (3) N.J.L. Roth & A.C. Craig; J. Phys. Chem. 78, 1154 (1974) | (4) R. Koenig, et al.; J. Luminesc. 9, 113 (1974) | (5) T.J.B. SIMONS; Nature 264, 467 (1976) | (6) C. Montecucco, et al.; Biochim. Biophys. Acta Biomembr. 552, 552 (1979) | (7) M.O.K. Enkvist, et al.; Brain Res. 462, 67 (1988) | (8) F. Bailey, et al.; Bioelectrochem. Bioenerg. 21, 333 (1989) | (9) G.M. Bilmes, et al.; J. Phys. Chem. 93, 6696 (1989) | (10) J.R. Bunting, et al.; Biophys. J. 56, 979 (1989) | (11) M.F. Hare & W.D. Archison; J.Neurochem. 58, 1321 (1992) | (12) P. Vaveliuk, et al.; J. Phys. Chem. 100, 11630 (1996) | (13) J.L. Seifert, et al.; JACS 121, 2987 (1999) | (14) A. Tomlinson, et al.; Chem. Phys. 325, 36 (2006) | (15) Y. Kawabe & S. Kato; Dyes Pigm. 95, 614 (2012)